Here are but a few benefits of the Cannabis plant for different symptoms of illnesses:
*Endoca does not state that CBD’s cure any types of disease, illnesses or ailments. Endoca FAQ
FOOD AND DRUG ADMINISTRATION (FDA) DISCLOSURE
These statements have not been evaluated by the FDA and are not intended to diagnose, treat or cure any disease. Always check with your physician before starting a new dietary supplement program.
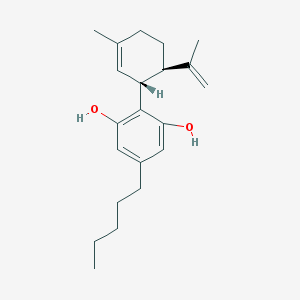
* Cannabidiol (CBD) is a natural constituent of hemp oil.
There is a very helpful video about the review of Endoca and the process in which it is extracted.
- To understand more about the Cannabis plant and it’s properties here is a great book about Cannabis Botany
- Another growing book that is helpful to understand the difference between indoor and outdoor cultivation.
- A study from the University of Oregon in 2008-09
There are doctors who are recommending healthy foods as a prescription over pharmaceuticals
“Although it was Hippocrates, the father of modern day medicine, who so famously stated “Let food be thy medicine, and medicine thy food,” few conventional doctors take seriously those words or wisdom and make wholesome, nutritious living the foundation of their patient’s treatment plan.
But in Houston, Texas, a progressive doctor has begun prescribing fruits and vegetables instead of pharmaceutical drugs, as he and many others believe nourishing food is an essential requirement for becoming ‘well’.
After years of treating patients’ modern-day ails, such as Diabetes and high blood pressure, Dr. Garth Davis discovered that diet and lifestyle are truly the best tools for helping a body become vibrantly well.“
Breast cancer Dr. David Gorski has serious concerns about the several claims that Cannabis cures cancer and shows his valid concerns as well as cites his sources to his findings. (This is not a PRO Cannabis article for the “curing of cancer” like some mainstream pro-medical marijuana industry entities. This is an education into how important the Rick Simpson Method of Cannabis extraction is to achieving the highest profile of helpful isomers like CBD & CBD (a) as well as delta-9-tetrahydrocannabinol for an equal ratio for maximum bioavailability in order to effectively treat cancer with cannabis.
- There are a growing number of scientists and doctors who are admitting that Cannabis has several medicinal uses in the treatment of Cancer and other terminal illnesses.
We believe that studies on cannabinoids and herpesviruses are important to continue because there are obvious potential benefits. Better understanding may lead to the development of specific non-psychoactive drugs that may inhibit reactivation of oncogenic herpesviruses. –
Delta-9 tetrahydrocannabinol (THC) inhibits lytic replication of gamma oncogenic herpesviruses in vitro - A Federal Study in 1981 has concluded that Cannabis is effective in treating anxiety.
Antibacterial Cannabinoids from Cannabis sativa - According to the American Epilepsy Society in (Abst. 3.397), 2015 from the Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: Novel anti-tumor mechanisms of Cannabidiol in breast cancer: -that CBD significantly inhibits epidermal growth factor (EGF)-induced proliferation and chemotaxis of breast cancer cells. Further studies revealed that CBD inhibits EGF-induced activation of EGFR, ERK, AKT and NF-kB signaling pathways as well as MMP2 and MMP9 secretion. In addition, we demonstrated that CBD inhibits tumor growth and metastasis in different mouse model systems. Analysis of molecular mechanisms revealed that CBD significantly inhibits the recruitment of tumor-associated macrophages in primary tumor stroma and secondary lung metastases.
- Efficacy and Safety of Epidiolex (Cannabidiol) in Children and Young Adults with Treatment-Resistant Epilepsy: Update from the Expanded Access Program – Cannabidiol (CBD) is the most abundant non-psychoactive cannabinoid in the cannabis plant. Animal studies demonstrate anticonvulsant efficacy in multiple species and models. Anecdotal reports suggest efficacy in children with treatment-resistant epilepsies (TRE), including Dravet Syndrome (DS) and Lennox-Gastaut Syndrome (LGS). We report current results in our expanded access treatment program.Methods: Children and young adults with TRE in an expanded access compassionate use program for CBD were enrolled in a prospective observational study. During the 4 week baseline, parents/caregivers kept prospective seizure diaries of all countable motor seizure types.
Patients received a highly standardized pharmaceutical plant-derived, purified CBD. (Epidiolex: GW Pharma), at a gradually increasing dose from 2-5 mg/kg/day until intolerance occurred or a maximum dose of 25 mg/kg/day was achieved.Patients were seen at regular intervals of 2-4 weeks during the initial 12 weeks of therapy. Testing for hematologic, liver, kidney function and AED levels was performed at baseline, and after 4, 8 and 12 weeks of CBD therapy.Results: 261 patients received at least 3 months of treatment and had available data at last group data collection (136 (52%) were male; average age 11.8 years, range 4 months-41 years; average weight 38 kg; range 6.4-127). The most common diagnoses were DS (44; 17%) and LGS (40; 15%). The average # of concomitant AEDs was 3.0. After 3 months of therapy, the median overall seizure frequency reduction was 45.1% in all patients and 62.7% in DS patients. For LGS patients, the median reduction of atonic seizures from baseline was 71.1%. Among all patients, 47% had a ≥50% reduction in seizures.Seizure-freedom at 3 months occurred in 9% of patients and 13% of DS patients. Clobazam co-therapy was associated with a higher rate of treatment response (≥50% convulsive seizure reduction): 57% v. 39%; this may reflect elevations in the desmethyl clobazam metabolite. Safety data from 313 patients representing 180 patient years was available at 16 sites. Adverse events in ≥10% of patients included somnolence (23%), diarrhea (23%), fatigue (17%), decreased appetite (17%), convulsions (17%) and vomiting (10%). 14 patients (4%) had an adverse event leading to discontinuation of CBD. 36 patients (12%) withdrew primarily due to lack of efficacy. Serious Adverse Events (SAEs) were reported in 106 patients (34%), including 7 deaths, none of which were considered treatment-related. 16 patients (5%) had SAEs that were considered treatment-related, including altered liver enzymes (4 pts; all were also on valproate and clobazam), status epilepticus/convulsion (4), diarrhea (4), decreased weight (3), thrombocytopenia (1), and others.Conclusions: These results from an uncontrolled study support the animal studies and prior reports showing that CBD may be a promising treatment for TRE and it is generally well-tolerated in doses up to 25mg/kg/day. Epidiolex is now being investigated in randomized controlled studies in DS and LGS.